An Anatomic Appraisal of Biplanar Muscle-Splitting Breast Augmentation
An Anatomic Appraisal of Biplanar Muscle-Splitting Breast Augmentation
Abstract
Background: Biplanar muscle-splitting (BMS) breast augmentation is a relatively new technique for which the safe regions of dissection have not been delineated.
Objectives: The authors performed cadaver dissections to elucidate the surgical anatomy of the BMS pocket and to infer the safety of this method.
Methods: The breasts and chest regions of 5 female cadavers were dissected to identify anatomic landmarks and to ascertain the optimal split site in the pectoralis major. CS was defined as the lateral junction of the middle and caudal one-third of the sternum, and the sternal index was defined as the ratio of the length of the sternum to the distance from CS to the most medial major nerve branch.
Results: Initiating the muscle split at CS is likely to avoid nerve injury. The mean distance from CS to the most medial nerve branch was 15.36 cm. The sternal index is a reproducible marker of the extension of the nerve branches in relation to chest size. The sternal length and the cranio-caudal length of the pectoralis major were similar, enabling reliable planning of the muscle split site.
Conclusions: If dissection is limited to the safe regions delineated herein, MS breast augmentation is likely to be a safe procedure for most patients. By maintaining the connections between the pectoralis major and its origins, a breast deformity associated with muscle contraction may be avoidable.
Introduction
Biplane muscle-splitting (BMS) breast augmentation is a relatively recent addition to the array of mammaplasty tech-niques. Although this method has become popular since its introduction by Baxter1 and Khan,2 an anatomic basis for safely splitting the pectoralis major has not been described. We sought to delineate the surgical anatomy of the BMS pocket that confers the aesthetic benefits of this method without potentially damaging potential morbidity of the pectoral nerves injury.
METHODS
Study Design
The breasts of 5 formalin-fixed female cadavers (n = 10 breasts) with intact chest walls and trunks were dissected at the Newcastle University Department of Anatomy (Newcastle upon Tyne, England) from April 2015 to June 2015. The dis-sections were performed in the Anatomy and Clinical Skills Centre at Newcastle University (Newcastle upon Tyne, England UK) following institutional approval from the Director of Anatomy and Clinical Skills and the Human Tissue Act Designated Individual (Dr Debra Patten). This study was con-ducted in accordance with guidelines set forth in the Declaration of Helsinki.
Dissection Procedures
Surgical Dissection of the BMS Pocket
The sternal outline was marked on the skin, and the lateral junction of the middle and caudal one-third of the sternum (CS) was marked with a horizontal line across the width of the sternal body (Figure 1). The planned implant position, based on characteristics of the native breast and typical implant widths, was marked. An inframammary incision was made and was carried perpendicular to the skin onto the pectoral/rectus fascia with monopolar diathermy. The gland was lifted from the chest wall to 2 cm beyond CS medially, and subglandular dissection was performed superolaterally from this point in the direction of the muscle fibers toward the lateral extent of the desired pocket.
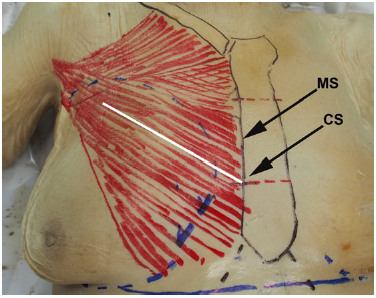
Figure 1. Markings before dissection of this 89-year-old female cadaver. The sternum and clavicle are marked in black, the pectoralis major is marked in red, and the gland boundaries and inframammary folds are in blue. The white line depicts the path of a biplane muscle split. CS, lateral junction of the middle and caudal one-third of the sternum. MS, midpoint of the sternum at its lateral border. The mean distance to the most medial nerve branch was consistently shorter from MS than from CS.
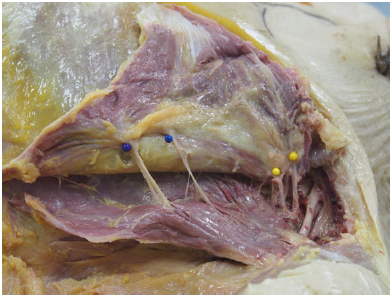
Figure 2. Right lateral view of a pectoral dissection in this 89-year-old female cadaver. The pectoralis major and minor have been detached superolaterally, and the entry sites of major nerves into the pectoral musculature are marked with pins.
The muscle split dissection then begins medially to the lateral edge of the sternum, where the muscle fibers are split with forcep diathermy. The muscle was split laterally along the path of the fibers to align with the subglandular dissection. The submuscular pocket then was created by lifting the split muscle adjacent to the lateral sternum and applying monopolar cautery in the submuscular plane to accommodate the implant. Meticulous care was required medially to avoid the internal mammary perforators. For implant placement, the muscle was not detached medially.
Cadaveric Dissection of the Pectoral Musculature and Nerves
The skin and subcutaneous fat were removed from the ante-rolateral chest to expose the pectoral region, including the clavicle, sternum, and acromion. The lateral insertion sites of the pectoralis major and minor were divided and reflected medially to expose the major vessels and pectoral nerves. The pectoral nerves and branches then were dissected istally into the pectoral muscles. The entry points of these branches into the pectoralis major were marked with pins on the anterior surface (Figure 2). Intramuscular branching pat-terns were not dissected. The pectoral muscles then were meticulously split in a medial-to-lateral fashion, stopping at the most medial point at which the nerve branches entered the deep surface of the pectoralis major. Several incisions were made into the pectoralis major to determine the optimal position of the muscle split. This was initially done on a separate pilot cadaver where different splits at incre-ments from the caudal sternum were attempted.
Cadaveric Assessment of the Pectoral Nerve Anatomy
All measurements were made from fully exposed bony landmarks with flexible measuring tape (millimeter-level precision) to accurately contour to the chest wall. The courses and precise locations of the major branches of the medial and lateral pectoral nerves were dissected and marked directly on the pectoralis major muscle. Specific an-atomic landmarks were designated, including CS, the mid-point of the sternum at its lateral border (MS), the sternal length (SL), and the length of the medial origin of the sternal head of pectoralis major (Figure 1). Measurements were taken from these landmarks to the points at which nerve branches pierced the pectoralis major.
The ratio of the length of the sternum to the length of the pectoralis major (ie, the pectoralis major index) and the ratio of the length of the sternum to the distance from CS to the most medial major nerve branch (ie, the sternal index) were determined. These data were compared across cadav-ers to ascertain whether point CS is a safe corridor for the muscle split that would avoid overt injury to the neural anatomy and achieve the aims of this approach.
Statistical Analyses
Paired t tests were performed using SPSS software, version 19 (IBM Corporation, Armonk, NY). Statistical significance was defined as P < .05.
RESULTS
The mean age of the cadavers was 89 years (range, 78-101 years). Surface landmarks and underlying structures de-scribed herein are depicted in Figure 1; nerve dissections are presented in Figure 2. An appropriate splitting pattern was ascertained by dividing the muscle incrementally from the most caudal portion of the sternal body. A muscle split originating from the caudal quarter of the sternum caused the caudal portion of the pectoralis major to detach from the rest of the muscle, thus denervating it. A muscle split cranial to CS intersected with major nerve branches within a short dissection distance, thereby limiting the volume of the submuscular pocket. The shortest distance from CS to the penetration of the first major branch of the lateral or medial pectoral nerve into the pectoralis major was longer than the corresponding distance from MS (mean distance, 15.36 cm vs 13.37 cm, respectively; CS range, 14.3-16.8 cm). The distances to the first major branch increased from caudal to cranial positions, from the lateral edge of the sternum.
The mean distance from the lowest point of the infra-mammary fold to the most medial pectoral nerve branch was 15.6 cm (range, 13.4-17.5 cm) (Figure 1). The sternal length correlated with the height of the pectoralis major in the craniocaudal axis at its medial origins. There were no sig-nificant differences between the lengths of the sternum and pectoralis major (P > .05) or between the sternal length and distance to the first major nerve branch (P > .05) (Table 1). A muscle split from CS did not detach the inferior part of the pectoralis major in any of the dissections.
DISCUSSION
The ideal pocket for breast augmentation would serve various purposes: to conceal the prosthesis, especially super-omedially; avoid a deformity associated with pectoral con-traction; preserve function of the pectoral girdle; preclude complications related to the capsule; and prevent injury to the nerves and chest wall. Nerve injury could result in atrophy of some or all of the pectoralis major, which could detract from the aesthetic result.
Dynamic Breast Deformity
Baxter1 and Khan2 proposed splitting the pectoralis major as a means to avoid a wide-cleavage deformity (ie, lateral displacement of the implants) and a so-called dynamic breast deformity (DBD; ie, implant displacement upon muscle contraction). The disruption of origins or insertions of muscles can produce a DBD with isometric contraction. The DBD remains an unfavorable aesthetic result following partial submuscular augmentation in its various forms. The patient should be informed preoperatively that DBDs are an anticipated consequence of submuscular implantation; oth-erwise, DBD could be viewed as a complication by the patient. Few studies have addressed the natural history of DBDs, possibly owing to excellent preoperative counseling and patient understanding that certain movements will produce breast distortion postoperatively.
With the BMS technique of breast augmentation, the implant and capsule could conceivably be pushed down-ward from muscle activity, but a noticeable DBD is unlikely because no muscle is liberated from its origin. We believe that most patients would tolerate mild, if any, downward displacement of their implant versus a DBD, however we have not objectively defined this yet. We are yet to encoun-ter a DBD in our unpublished data of 50 patients who un-derwent primary or revisional BMS augmentation.
Unlike partial submuscular implant placement, the BMS procedure preserves the origin and insertion of the muscle fibers covering the implant, which may preserve function of the pectoralis major better than methods that involve detachment of the inferomedial aspect of the muscle origin. In our experience, preserving the attachment of the pector-alis major to all of its origins and insertions is a means of preventing DBD. However, preservation of the pectoral nerves in general does not eliminate the possibility of DBD occurrence, and some surgeons have intentionally dener-vated the pectoralis major to treat contraction-related dis-tortion of submuscular implants.3
Spear et al4 asked independent observers to grade DBD in 40 patients who underwent a modified technique of dual-plane augmentation. Over half of this cohort were deemed to have at least a mild form of DBD. In addition, these researchers sent questionnaires to 195 patients to assess patient satisfaction and the development of DBDs. Of the 69 respondents, 12 (17.4%) had experienced moder-ate to severe distortion with activity, but only 1 patient noted that she would not recommend submuscular implant placement to others.4 However, this study had a low rate of patient response (35%), so sampling bias may have influ-enced these results.
Rationale for the BMS Approach
In our experience, DBDs are a common patient concern with submuscular augmentation, but few patients seek sur-gical revision. Those who do tend to be gym enthusiasts who are concerned that activation of the detached pectora-lis major draws attention to their breast augmentation. Although objective data are lacking regarding patient satis-faction with partial submuscular techniques, any technique that entirely avoids a DBD would yield superior aesthetic results.
Khan2 originally described a muscle split from the junc-tion of the middle and caudal third of the sternal origin on the pectoralis major that proceeded superolaterally along the muscle fibers. The author did not provide his reasoning for initiating the muscle split at this location, but this tech-nique avoids muscle division from its origins and the infra-mammary fold, thereby positioning the implant deep to the muscle superomedially and superficial to the muscle inferi-orly (Figure 3).2 In a retrospective analysis of 2026 patients who underwent augmentation mammaplasty by the same surgeon, Khan5 found that DBD is avoided using the BMS technique and can be used effectively to correct DBD in re-vision cases. We regard the BMS approach as potentially optimal for avoiding DBDs in primary augmentation and for correcting DBDs in revisional augmentation for patients who previously received implantation in a dual-plane pocket.1,6
Positioning the Muscle Split
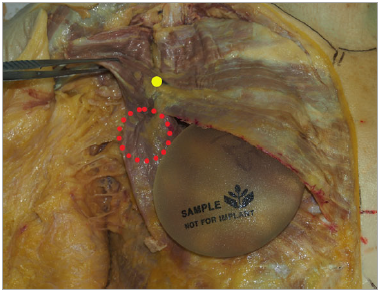
Figure 3. Placement of a round implant in a biplane
muscle-splitting pocket in this 89-year-old female cadaver. The upper medial portion of the implant is submuscular. The yellow dot indicates the entry site of the most medial nerve branch into the pectoralis major. There is no separation of the inferior portion of the muscle (depicted by a red circle), and the muscle is not detached from its medial origin. Inferior sepa-ration would occur if the split was initiated more caudally on the pectoralis major.
The results of our cadaver dissections indicate that major nerve injury and poor implant coverage would be likely if the initial muscle split is made cranial to the junction of the middle and lower thirds of the sternal origin of the pectora-lis major. The distance from MS to the most medial nerve branch was consistently shorter than the distance from CS to this branch (Figure 1), indicating that a more cranial muscle split would likely encounter major nerves. A more caudal muscle split would result in denervation of the infe-rior portion of the pectoralis major. The medial extent of the pocket also must be considered for BMS augmentation. Medial implant malposition is a relatively rare phenomenon for which the etiology may be surgical or anatomic.7 Overzealous submuscular dissection in the medial direc-tion eliminates buffer space between the implant pockets and may result in symmastia.
Biplane muscle splitting does not involve division of the medial (ie, sternal) origins of the pectoralis major; theoreti-cally, this should preserve the isometric contractile function of the muscle. However, this approach does involve divi-sion of some costal fibers, and ligation may be required for internal mammary perforators that emerge through inter-costal spaces. Although they decrease in diameter at more caudal locations, these perforators emerge up to 1.4 cm lat-erally from the lateral sternal border.8 In our experience, the internal mammary perforators are a reliable marker of the medial boundary of the pocket.
Variations of the Pectoral Musculature: Muscle Slips
The pectoral girdle is a region of anatomic variability. The results of large series of cadaver dissections have indicated variations in costal origins, insertion sites of muscle slips, and lateral interconnections between the pectoralis minor and major.7,9-11 Variations in the latter 2 structures have im-plications for breast augmentation. Accessory fibers exist between the pectoralis major and minor and join portions of these muscles laterally and caudally.9,12 This incomplete muscle separation was observed in 8 of 202 pectoralis major specimens (4%)12 andisbelievedtooccur duringembryo-genesis.9 A topographic analysis of the interconnections of the pectoralis major and minor fibers is beyond the scope of this study. However, such interconnections would compli-cate the subpectoral approach, which begins at the free inferi-or border of the muscle. Although interconnecting fibers are believed to predominate inferolaterally on the chest wall,12 they also may occur in the submuscular region constituting the lateral limit of a BMS dissection. This topic warrants further scrutiny, particularly because incomplete muscle sep-aration could contribute to medialization of the implant in rare cases if not recognized and divided appropriately.
Additional slips of muscle related to the pectoralis major are relatively common. In a retrospective review, Moliver et al7 identified patients who underwent revisional surgery to treat symmastia or medial implant malposition that was associated with pectoralis slips secondary to subpectoral breast augmentation. Perrin10 found that distinct muscular elements originated immediately inferior and parallel to the pectoralis major in 21% (n = 29) of dissections. These slips of muscle entered the pectoralis major tendon just proximal to the humeral insertion and were called epigas-tric slips.10 Less common types of slips were into the latissi-mus dorsi or the medial epicondyle of the humerus and were often bilateral.10 Unlike with epigastric slips, the activ-ities of these slip variants would not be in concert with the pectoralis major.
We posit that Perrin10 may have miscategorized fibers of the pectoralis major as frequent epigastric slips. An areolar plane exists between the clavicular and sternal heads of the muscle. If a similar plane exists between an epigastric slip and the sternal head, it may be unnecessary to distinguish these fibers from the pectoralis major given that they join the pectoralis tendon. Instead, the epigastric slip may be analogous to what some investigators and anatomists rou-tinely term the abdominal head of the pectoralis major.13
In practice, anomalies of the pectoral musculature can affect the lateral region of the pocket. Muscle slips may impede the creation of a standard submuscular pocket by requiring specific division during the lateral dissection. An epigastric slip would remain undisturbed on the chest wall during creation of a biplane pocket, however such a slip could adhere to the posterior surface of capsule (and implant). Contraction of this muscle slip could conceivably distort implant position, but whether this would be visible clinically is not known.5,6,14
Investigators who previously described cadaver dissec-tions to produce a BMS pocket by inframammary or trans-axillary approaches did not report whether they attempted to elicit a DBD postoperatively.1,2,14 The BMS method does not create loose muscle tissue that could attach to the capsule and/or lower pole of the gland and distort the soft tissues and implant. Instead, this procedure preserves the attachment of the stronger and more voluminous15,16 lower portions of the pectoralis major to the chest wall. The weaker cranial aspects of the muscle that cover the upper medial aspect of the implant could theoretically push the implant inferiorly. However, we were unable to elicit this effect with shoulder adduction and flexion thus far in clini-cal cases. The posterior adherence of the capsule to the chest wall superiorly could balance its adherence to the muscle inferiorly. In addition, the small anterior region in which the capsule contacts the muscle could translate to minimal effects on the implant with pectoral activation.
Study Limitations and Future Research Needs
Our study has several limitations, including a small sample size. Although we performed fewer dissections than others who evaluated this type of augmentation, we did not seek to comprehensively examine the gross anatomy of the pectoralis major and interacting tissues. Rather, we aimed to determine whether a reproducible safe region of pocket dissection could be defined. In addi-tion, we dissected cadavers of relatively advanced ages. Most women who seek primary breast augmentation are younger than the cadavers in our study. However, this discordance in age would likely be an issue for most cadaver studies. We did not delineate the intramuscular course of the nerve branches. We suspect that the BMS approach would sever small intramuscular branches, which could have varied, minor, or asymmetric effects on denervation and muscle atrophy.
Despite our small sample size, we identified a compel-ling consistency in anatomic landmarks that warrants further clinical investigation. The length of the sternum cor-related with the length of the pectoralis major, indicating that landmarks on the sternal surface are reproducible. Our data indicate that a patient with a longer sternum also would likely have a longer pectoralis major at its medial origin in the craniocaudal axis. We observed a consistent relationship between sternal length and the distance from CS to the most medial major branch of the pectoral nerve (ie, the sternal index). Similarly, the distance to the nerves may be predicted from the origin of the muscle split. The mean distance was 15.4 cm from CS, and the most medial nerve branch was a consistent distance from CS (Table 1).
The lateral pectoral nerve follows a predictable course to its entry into the pectoralis major. Macchi et al17 demon-strated that this nerve does supply all portions of the pec-toralis major. These authors17 also observed connections between the pectoral nerves around the pectoralis minor, supporting findings of other anatomic studies.4 Multiple branches of the medial pectoral nerves innervate the pec-toralis major; the lateral extents of these nerves consistently supply the lateral caudal portion of the pectoralis major.18 We examined only the most medial nerve branch that would be encountered in BMS augmentation, given this well-documented variability in innervation.17,18
To our knowledge, there are no studies in which electro-myographic analyses were performed to map the contribu-tions of the nerve branches and their effects on the pectoral muscles. Therefore, we cannot rule out the possibility of BMS augmentation resulting in partial denervation of the caudal pectoralis major. Because the BMS approach pre-serves the major nerves and avoids division of the muscle from its sternal origins, we expect that it would be superior to other submuscular techniques in terms of preserving muscle function.
Our findings support that a muscle split from CS would maintain the lateral continuity of the pectoralis major (Figure 2) and would not disrupt the major pectoral nerve branches at their entries into the pectoralis major and minor. The most medial nerve branch could only be damaged at its site of penetration into the pectoralis major if a pocket was created to accommodate a very large implant (ie, >15 cm wide) in a small chest. In our experi-ence, this has not been a limitation because most patients do not request a vastly disproportionate implant size. Moreover, even the largest available implants (round or otherwise shaped) would be more easily housed in a BMS pocket because the pocket would only need to accommo-date the upper medial pole of the implant.
CONCLUSIONS
In this cadaver study, a BMS approach initiated at CS afford-ed a safe area of dissection for the creation of a pocket, without damaging major pectoral nerve branches. A BMS approach in our early experience avoids DBD and so we wanted to define the surgical anatomy. To our knowledge, the current study is the first to address whether the biplane pocket potentially avoids alternate morbidity such as pecto-ral nerve injury. This is an important consideration because atrophy of the denervated muscle located inferiorly on the chest wall could yield implant malposition over time and therefore may not improve on the development of DBD using other existing sub-muscular techniques. Further clini-cal study is warranted to objectively evaluate the natural history of DBDs and to ascertain whether the BMS tech-nique is associated with any long-term functional impair-ment that may be deemed worse than DBD.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Acknowledgements
The authors would like to thank Mr Brian Thompson and Dr Debra Patten of the Newcastle University Department of Anatomy for their help in carrying out this work.
REFERENCES
- Baxter RA. Subfascial breast augmentation: theme and variations. Aesthet Surg J. 2005;25:447-453.
- Khan UD. Muscle-splitting biplane breast augmentation: a new pocket in a different plane. Aesthetic Plast Surg. 2007;31:553-558.
- Maxwell GP, Tornambe R. Management of mammary subpectoral implant distortion. Clin Plast Surg. 1988;15 (4):601-611.
- Spear SL, Schwartz J, Dayan JH, Clemens MW. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 2009;33:44-48.
- Khan UD. Muscle-splitting, subglandular and partial sub-muscular augmentation mammoplasties: a 12-year retro-spective analysis of 2026 primary cases. Aesthetic Plast Surg. 2013;37:290-302.
- Khan UD. Dynamic breasts: a common complication following partial submuscular augmentation and its cor-rection using the muscle-splitting biplane technique. Aesthetic Plast Surg. 2009;33:353-360.
- Moliver CL, Sanchez ER, Kaltwasser K, Sanchez RJ. A muscular etiology for medial implant malposition following subpectoral augmentation. Aesthet Surg J. 2015;35:NP203-NP210.
- Gillis JA, Prasad V, Morris SF. Three-dimensional analysis of the internal mammary artery perforator flap. Plast Reconstr Surg 2011;128:419e-426e.
- Lewis WH. Observations on the pectoralis major muscle in man. Johns Hopkins Hosp Bull. 1901;12:172-177.
- Perrin JB. Notes on some variations of the pectoralis major with its associate muscles. J Anat Physiol. 1871;5:233-240.
- Anson BJ, Jamieson RW, O’Conor VJ Jr, Beaton LE. The pectoral muscles; an anatomical study of 400 body-halves. Q Bull Northwest Univ Med Sch. 1953;27:211-218.
- Sanchez ER, Sanchez R, Moliver C. Anatomic relation-ship of the pectoralis major and minor muscles: a cadav-eric study. Aesthet Surg J. 2014;34:258-263.
- Sim HB, Hwang K, Huan F, et al. Anatomy and tensile strength of the abdominal head of the pectoralis major muscle in relation to transaxillary breast augmentation. Aesthetic Plast Surg. 2013;37:359-363.
- Stumpfle RL, Pereira-Lima LF, Valiati AA, Mazzini GS. Transaxillary muscle splitting breast augmentation: experience with 160 cases. Aesthetic Plast Surg. 2012;36: 343-348.
- Fung L, Wong B, Ravichandran K, Agur A, Rindlisbacher T, Elmaraghy A. Three-dimensional study of pectoralis major muscle and tendon architecture. Clin Anat. 2009;22:500-508.
- Ackland DC, Pak P, Richardson M, Pandy MG. Moment arms of the muscle crossing the anatomical shoulder. J Anat. 2008;213:383-390.
- Macchi V, Tiengo C, Porzionato A, et al. Medial and lateral pectoral nerves; courses and branches. Clin Anat. 2007;20:157-162.
- Prakash KG, Saniya K. Anatomical study of pectoral nerves and its implications in surgery. J Clin Diagn Res. 2014;8:AC01-AC05.